Clinical Study
AC102: A New Drug to Treat Sudden Hearing Loss
There is currently no approved medication for the treatment of Sudden Sensorineural Hearing Loss (SSNHL). Clinical practice guidelines often recommend the use of glucocorticoid. Unfortunately, there is only weak evidence that this standard-of-care results in a clinically relevant benefit to the patient. Namely, the US Clinical Practice Guideline for the Treatment of Sudden Hearing Loss states that for SSNHL:
corticosteroid treatment is one of the few treatment options that has any data showing efficacy, although even those data are somewhat equivocal.1
AC102 Outperforms Glucocorticoids in Preclinical Studies
In preclinical studies of hearing disorders, AC102 significantly outperformed glucocorticoid treatment and resulted in almost complete normalization of hearing thresholds.
AC102 is Safe and Well-Tolerated in Healthy Volunteers
After state-of-the-art research on the properties of AC102 to support its safety for use in humans, it was tested in a first-in-human clinical trial. This Phase 1 study was successfully completed in 2021 and demonstrated that AC102 was both safe and well tolerated by healthy volunteers when administered directly into the middle ear.
Efficacy of AC102 is Evaluated in Patients with Hearing Loss
AC102 is currently being evaluated in patients in a randomized, blinded Phase 2 clinical trial. The trial is enrolling approximately 200 patients with moderately-severe to profound Idiopathic (of unknown cause) Sudden Sensorineural Hearing Loss. The trial will compare the efficacy, safety, and tolerability of AC102 with a standard treatment with oral glucocorticoids (see Figure 1).
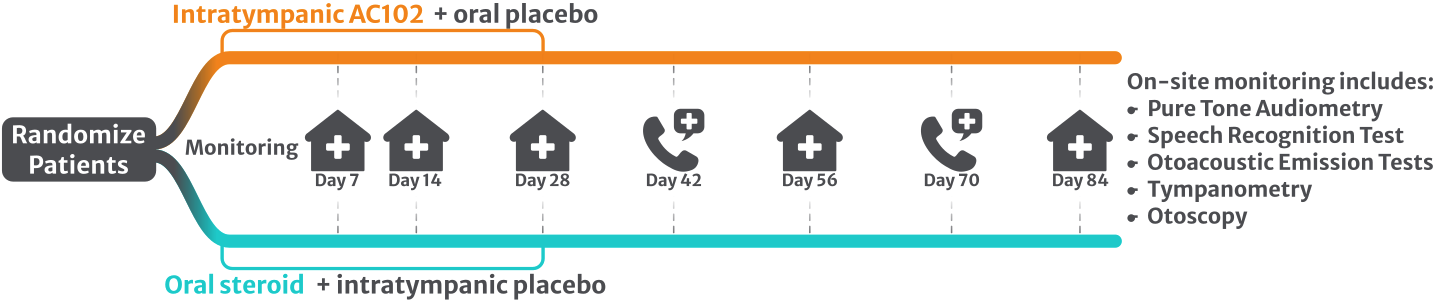 Figure 1: Clinical trial design includes multiple clinic visits and calls to monitor patients’ audiological parameters and general health throughout the 3 month study period.
Figure 1: Clinical trial design includes multiple clinic visits and calls to monitor patients’ audiological parameters and general health throughout the 3 month study period.
The trial design ensures that all patients will receive a treatment, either glucocorticoids (a standard of care) or the novel compound AC102. AC102 is administered into the patient’s middle ear, which optimizes local drug concentration and minimizes systemic side effects. Importantly, the treatment needs to be administered within 5 days of the onset of symptoms (see Figure 2).
 Figure 2: Main inclusion and exclusion criteria for the clinical trial. To note are the symptom onset no longer than 120 hours before treatment and that pre-treatment with glucocorticoids is excluded. Idiopathic hearing loss = hearing loss of unknown cause.
Figure 2: Main inclusion and exclusion criteria for the clinical trial. To note are the symptom onset no longer than 120 hours before treatment and that pre-treatment with glucocorticoids is excluded. Idiopathic hearing loss = hearing loss of unknown cause.
Patient care after treatment is also an important aspect in this clinical trial as it includes 5 clinic visits and 2 phone calls. The patients’ general health (laboratory parameters, vital signs, ECG, etc.) and audiological parameters will be closely monitored. ENT physicians at more than 45 European study sites are involved based in Austria, Czech Republic, Germany, Netherlands, Poland and Serbia (see Figure 3). This strong commitment underscores the high medical need to find an effective treatment for Sudden Hearing Loss.
If you are interested in participating in our clinical trial, please click on your country on the map to find and contact your nearest study site: