Information for Physicians
AC102 Clinical Study
Comparison of the new compound AC102 with prednisolone with regard to its efficacy in patients with idiopathic sudden sensorineural hearing loss (ISSNHL)
Main Inclusion Criteria
-
- Females or males, 18 to 80 years of age
- Onset of unilateral ISSNHL 24 to 120 hours prior to first study treatment at the study site
- Absolute air conduction hearing threshold of at least 65 dB, but less than 100 dB (average of three most affected consecutive frequencies between 0.25 and 8 kHz)
- Relative hearing loss of at least 40 dB compared to the unaffected ear at the three most affected frequencies
Main Exclusion Criteria
-
- Acute hearing loss from noise trauma, barotrauma or head trauma
- History of radiation-induced hearing loss, fluctuating hearing, endolymphatic hydrops or Menière’s disease
- History of chronic inflammatory, suppurative ear disease or cholesteatoma
- Uncontrolled blood pressure, uncontrolled diabetes
Prohibited Medications or Treatments
-
- Any steroid therapy
- Radiation therapy in the head or neck area
- Any therapy with ototoxic drugs (e.g. aminoglycosides, cisplatin)
- Systemic or intratympanic treatment with steroids or hyperbaric oxygen
- Immunosuppressive substances, including ciclosporin
Next Steps
If the patient meets the above criteria and is willing to consider a study participation and you wish to offer him/her the opportunity to present themselves to our participating study center, please refer him/her to us. In case the patient is successfully included into the trial, a compensation of 100 € will be made to you for your referral efforts. After the study the patient will come back to your practice.
AudioCure Pharma GmbH · Schlegelstr. 9 · 10115 Berlin · Germany · Email: info@audiocure.com · Phone: +49 (30) 221 8397 0
Background Information
AC102
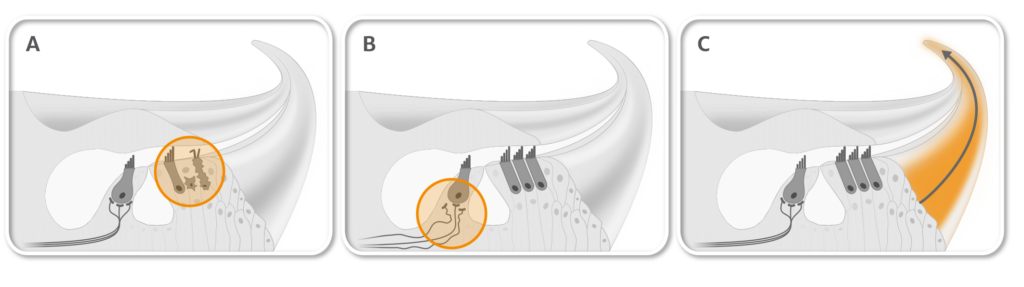
AC102 is a novel compound in clinical development for acute hearing loss which clearly outperformed corticosteroid treatment in preclinical animal studies. A single intratympanic application of AC102 improved hearing to almost normal levels across all frequencies. AC102 increased the survival of sensory hair cells (A) and restored synaptic connections to the auditory nerve (B) (see figure below). Moreover, AC102 distributes well through the whole tonotopic axis of the cochlea (C).
Test Substance and Safety
AC102 is formulated in a thermosensitive hydrogel that is administrated via a standard intratympanic injection into the middle ear. The individual composition of the formulation allows for a sustained drug release and exposure of the target inner ear tissue. Advantages of the local drug application are high therapeutic levels with almost no systemic side effects.
In a first-in-human trial, AC102 demonstrated its safety and tolerability in healthy volunteers. Adverse events were nearly all mild and of short duration. Based on its safety profile, AC102 is now being tested for efficacy in patients.
Design of the Clinical Trial
In the present Phase II clinical trial, a single intratympanic injection of AC102 will be administered to the affected ear within 24 and 120 h after hearing loss onset. Due to the severity of the disease, AC102 will be compared against the standard of care medication, steroids (oral prednisolone 60 mg/day over 14 days followed by tapering over 10 days). The study will be conducted in a randomized, blinded manner. All patients will receive an intratympanic injection and tablets – one will be the active medication the other the corresponding placebo. Approximately 210 patients will be enrolled in 8 countries. Efficacy will be evaluated by pure tone audiometry and speech recognition tests. A salvage therapy can be offered to patients with no improvement in hearing after 4 weeks. The total study duration is 12 weeks with 6 on-site visits.
The Company
AudioCure Pharma GmbH is a team of innovative scientists pioneering the discovery and development of novel treatments for hearing disorders. Based on decades of research of our founder, Prof. Hans Rommelspacher MD, we identified highly promising new substances and combine them with advanced drug-delivery formulations with the goal to improve patients’ quality of life in diseases with a high unmet medical need.